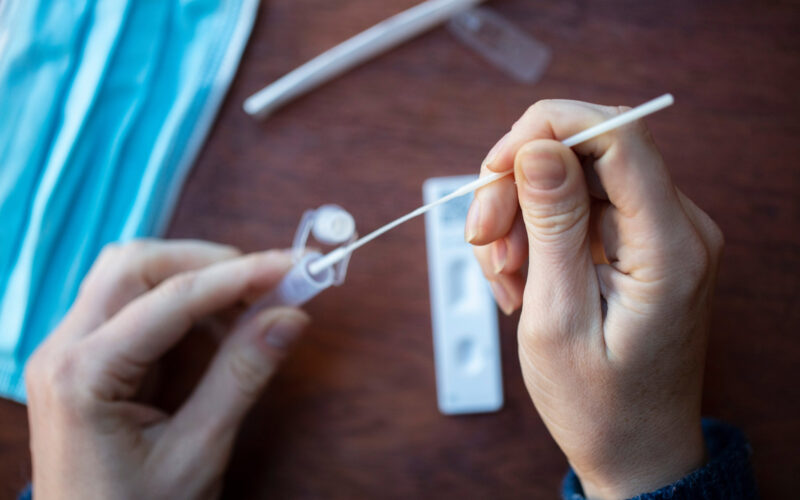
Each year, more than 6 million people are infected with tuberculosis, and globally there are more than 1 billion people suffering from tuberculosis. The tuberculosis pandemic poses a serious threat, but there is hope. The Danish biotech startup company, VPCIR Biosciences, has developed a new, non-invasive weapon to fight this dreaded disease.
Tuberculosis can be both prevented and cured, but it requires early detection and treatment. Unfortunately, this is not happening sufficiently, resulting in up to 1 million people dying from the disease each year. This could have been avoided if the disease had been detected and treated with antibiotics in time. One of the major challenges is that the existing test methods are not easily available in those developing countries, where tuberculosis is most prevalent.
Traditionally, tuberculosis is diagnosed by analysing mucus from the lungs, a method that is expensive, requires a lot of equipment, and is time-consuming. In addition, invasive interventions may be necessary if the patient cannot cough up mucus himself.
But these challenges may be a thing of the past if you ask the reseachers at VPCIR Biosciences. The company’s vision is to develop a new and more sensitive diagnostics technology for commercial use. Their commitment to their research is a step towards a future where tuberculosis is no longer af global threat.
Lenghty research process aimed at commercial use
The development of VPCIR’s technology has been going on for many years. Already in the year 2000, CSO and co-founder of VPCIR, Birgitta R. Knudsen, started her basic research within detection of infectious diseases. It took time to adjust the techology, so it could work in a stable and secure manner. After 13 years of research, Birgitta and the other co-founders, Marianne Hede, Megan Yi-Ping Ho, Magnus Stougaard, and Jan Mousing, started focusing specifically on tuberculosis. However, the meticulous research and the wait was worth it.
Our technology is non-invasive, since it detects the patogenes in saliva. And it is more sensitive. This means that the detection limit is better with our method than for competing methods
Birgitta R. Knudsen, CSO and co-founder of VPCIR Biosciences
VPCIR’s technology also aims to make it easier to get reliable test results in remote areas of delevoping countries. There is still potential for further development of the technology.
”The next step is to make the technology robust enough to be developed into commercial medical equipment. One thing is to make it work in a research lab. Something else is to make it easy to use for others, even people who do not have a long education within this field,” explains Jørgen Schøller, who is CEO at VPCIR Biosciences. Jørgen continues:
”The aim is to develop a solution which makes it easy for health professionals to test the locals and get results on the spot.”
Expert knowledge optimised the patent process
In the past year, VPCIR was awarded Plougmann Vingtoft’s yearly donation, IP Matters, for their groundbreaking technology. The donation consists of IP counselling worth 50,000 DKK and is awarded to Danish startup companies which have a potential to influence one or more of the United Nations 17 Global Goals. In this context, VPCIR has received counselling from Jakob Schwalbe Lohmann, who is a European Patent Attorney at Plougmann Vingtoft.

When Jakob was a PhD student, he worked with the early versions of the diagnostics technology, and he therefore possesses in-depth knowledge about VPCIR’s research areas. It is not always possible to expect that a patent consultant possesses an equally high degree of expertise, but the patent consultants at Plougmann Vingtoft always have a thorough knowledge of the technological area.
Jakob explains: ”It is important that the patent consultant has technical knowledge, so that we can quickly familiarise ourselves with the specific technology. The researchers are the experts, and the patent consultant can translate their knowledge into patent language.”
It has been a help to VPCIR that Jakob has had expertise within the technology. Birgitta says: ”It has optimised the process significantly to use Plougmann Vingtoft. There is no doubt that Jakob understands the technology thoroughly and it is easy to exchange ideas with him in connection the patent applications.”
Difficult balance between publication and patenting
Furthermore, Jakob has given advise to VPCIR concerning the complex balance between publication and patenting. As a university researcher, you are faced with a double obligation: to share your research with the world and at the same time avoid revealing too much about your invention before the patent application is filed.
Plougmann Vingtoft’s patent consultants have extensive experience with university based innovation – a category which VPCIR falls under. This experience was clear to the team at VPCIR:
The patent consultants at Plougmann Vingtoft are good at acting in this university associated environment, where you are constantly in a area of conflict between publication and patenting. They have been good at advising us about the timing of filing patent applications, and they have been very responsive with respect to filing applications in time – sometimes the day before publication.
Birgitta R. Knudsen, CSO and co-founder of VPCIR Biosciences
According to Jakob Schwalbe Lohmann, both the researchers and the technology transfer offices at the universities have in recent years developed a strong awareness of the patent process and have become good at finding the correct balance between publication and patenting. It is Jakob’s experience that the universities certainly do not limit the researchers’ ability to publish scientific articles, but it is more a matter of doing things in the right order, in order not to damage potential commercial interests.
It is encouraging to se how the universities become more aware of the patent process and work towards protecting innovation without limiting knowledge sharing.
At VPCIR the patent process is well under way, just as the journey to revolutionize the diagnostics of tuberculosis.
Facts about tuberculosis
- Spreading: Tuberculosis occurs globally, but most frequently in areas such as sub-Saharan Africa, Southeast Asia, and the western Pacific.
- Latent infection: The tuberculosis bacterium can lie latent in the body without visible symptoms. Preventive treatment can prevent the disease from breaking out.
- Lack of diagnosis: WHO estimates that almost 16% of those infected are not diagnosed – this corresponds to approx. 1.5 million people.
- Spread of infection: A tuberculosis patient infects an average of 10-15 others before the disease is detected and treated.
- Mortality: Nearly 1 million people died from tuberculosis in 2023, making it one of the world’s second deadliest infectious diseases.
- Treatment: Only two in five people with multi-resistant tuberculosis received treatment in 2022, and only one in two people with latent tuberculosis received preventive treatment.
Source: World Health Organization, WHO
Photo: Adobe Stock
What is IP Matters?
Each year until 2023, Plougmann Vingtoft will donate IP counselling worth 50,000 DKK to Danish startup companies, which we think can influence one or more of the United Nations 17 Global Goals.

Together with the entreprenurial network, Danish Business Angels (DanBAN), we select a winner with a convincing vision for a sustainable future. A company, which can get the most out of our knowledge about IP rights and our counselling about business development.
Those companies, which are awarded the IP Matters donation, will be in contact with a team of carefully chosen IP experts with specific knowledge within the business area in question. Find all our experts here, or send an e-mail to pv@pv.eu in order to find out more about what we can offer you and your company.